Quality by Design (QbD)
Quality by Design is described as an extensive, systematic product or development process in the pharmaceutical industry. It means that the whole product life cycle is already evaluated in an early stage of a product, taking into account scientific findings and risk assessment.
This systematic approach, including clearly pre-defined goals, focusses on process and product understanding as well as on process control. In doing so, the QdB method guarantees a higher product quality at minimized risk. It exceeds thereby the „minimally required procedure“ considerably and, nevertheless, enables shorter and more robust development processes.
A development process according to QdB for the pharmaceutical industry is divided basically into six phases. For all six phases, from product profile (Target Product Profile – TPP) via risk analysis and evaluation of critical quality attributes (QAs) to risk assessment, software-based tools for the execution and the documentation are needed.
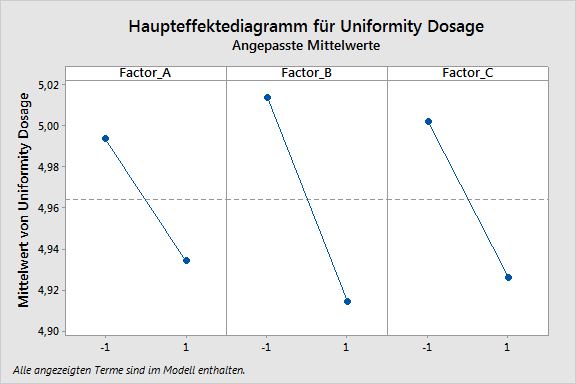
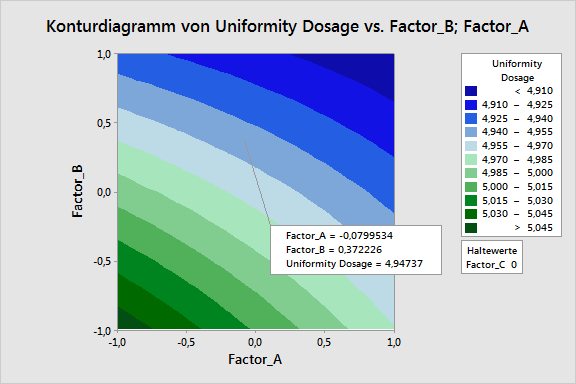
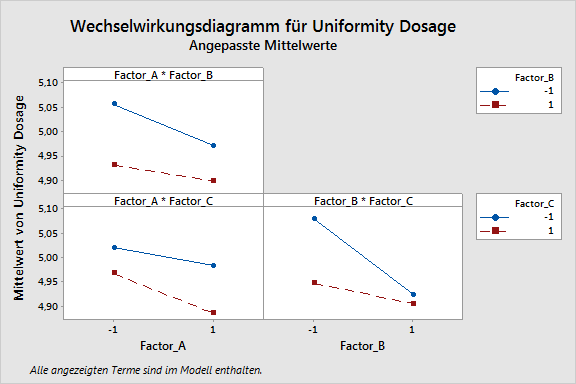
During all the phases, our customers are supported optimally by the products of Minitab Inc., especially the statistical software Minitab and Minitab Workspace, as well as by ADDITIVE‘s AQUA server. For example, it is possible to perform and document process maps, design and process FMEAs, as well as a complete quality function deployment (QFD) by using Minitab Workspace. A centerpiece of QbD is statistical safeguarding by means of the design of experiments (DoE), a function fully provided by Minitab, including response opimization.
Risk assessment as the last phase covers a permanent control process. Here, statistical analysis tools like control charts, shelf-life studies and capability studies can be used. These required statistical features are also available in Minitab. By using Minitab, you can create partially automated analyses and reports (with macro) and company-specific customizations. By using the AQUA server in combination with the software Minitab, you can create process dashboards and fully automated reports.